Aqueous Lead Ii Nitrate Formula
 | |
 | |
| Names | |
|---|---|
| IUPAC name Atomic number 82(II) acetate | |
| Systematic IUPAC name Pb(Ii) ethanoate | |
| Other names Plumbous acetate, Salt of Saturn, Sugar of Lead, Lead diacetate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.005.551 |
| EC Number |
|
| MeSH | lead+acetate |
| PubChem CID |
|
| RTECS number |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| InChI
| |
| SMILES
| |
| Properties | |
| Chemical formula | Lead(C2HiiiO2)2 |
| Tooth mass | 325.29 g/mol (anhydrous) 379.33g/mol (trihydrate) |
| Appearance | White powder or colourless, efflorescent crystals |
| Odor | Slightly acetic |
| Density | iii.25 one thousand/cm3 (20 °C, anhydrous) two.55 yard/cmiii (trihydrate) 1.69 grand/cmiii (decahydrate)[1] |
| Melting betoken | 280 °C (536 °F; 553 Thou) (anhydrous) 75 °C (167 °F; 348 1000) (trihydrate) decomposes[4] at ≥ 200 °C 22 °C (72 °F; 295 1000) (decahydrate)[1] |
| Boiling point | Decomposes |
| Solubility in water | Anhydrous: 19.8 yard/100 mL (0 °C) 44.31 k/100 mL (twenty °C) 69.5 thousand/100 mL (thirty °C)[two] 218.3 g/100 mL (l °C)[1] |
| Solubility | Anhydrous and trihydrate are soluble in alcohol, glycerol[ii] |
| Solubility in methanol | Anhydrous:[ii] 102.75 k/100 1000 (66.1 °C) Trihydrate:[3] 74.75 g/100 g (fifteen °C) 214.95 g/100 thousand (66.1 °C) |
| Solubility in glycerol | Anhydrous:[2] 20 g/100 k (15 °C) Trihydrate:[3] 143 one thousand/100 g (20 °C) |
| Magnetic susceptibility (χ) | −89.1·10−half-dozen cm3/mol |
| Refractive alphabetize (n D) | 1.567 (trihydrate)[1] |
| Structure | |
| Crystal structure | Monoclinic (anhydrous, trihydrate) Rhombic (decahydrate) |
| Thermochemistry | |
| Std enthalpy of | −960.9 kJ/mol (anhydrous)[2] −1848.6 kJ/mol (trihydrate)[3] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Neurotoxic, likely human carcinogen |
| GHS labelling: | |
| Pictograms |   [iv] [iv] |
| Bespeak discussion | Danger |
| Gamble statements | H360, H373, H410 [iv] |
| Precautionary statements | P201, P273, P308+P313, P501 [4] |
| NFPA 704 (burn down diamond) | two 1 i |
| Flash point | Not-combustible |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 400 mg/kg (mice, oral)[1] |
| LCLo (lowest published) | 300 mg/kg (domestic dog, oral)[5] |
| Related compounds | |
| Other cations | Lead(IV) acetate |
| Except where otherwise noted, data are given for materials in their standard country (at 25 °C [77 °F], 100 kPa). Infobox references | |
Lead(2) acetate (Lead(CH3COO)2), also known as lead acetate, lead diacetate, plumbous acetate, sugar of atomic number 82, lead sugar, salt of Saturn, or Goulard's powder, is a white crystalline chemical compound with a slightly sweet taste. Like many other atomic number 82 compounds, it is toxic. Lead acetate is soluble in water and glycerin. With water it forms the trihydrate, Lead(CHthreeCOO)2·3HtwoO, a colourless or white efflorescent monoclinic crystalline substance.
The substance is used equally a reagent to make other atomic number 82 compounds and as a fixative for some dyes. In depression concentrations, it is the principal active ingredient in progressive types of pilus colouring dyes.[half-dozen] Pb(Ii) acetate is likewise used as a mordant in textile printing and dyeing, and as a drier in paints and varnishes. It was historically used equally a sweetener and preservative in wines and in other foods and for cosmetics.
Production [edit]
Lead acetate tin be made past boiling elemental lead in acetic acrid and hydrogen peroxide. This method will also work with pb carbonate or pb oxide.
Pb(s) + HtwoO2(aq) + ii H+(aq) → Pb2+(aq) + two HtwoO(50)
Pb2+(aq) + 2 CHthreeCOO−(aq) → Atomic number 82(CH3COO)2(aq)
Lead(Ii) acetate can also be made via a single displacement reaction betwixt copper acetate and pb metal:
Cu(CH3COO)ii + Pb → Cu + Pb(CH3COO)two
Structure [edit]
The crystal structure of anhydrous lead(2) acetate has been described as coordination polymer. In comparison, lead(II) acetate trihydrate's structure is a 1D coordination polymer.[7] In the trihydrate, the Pbii+ ion'southward coordination sphere consists of ix oxygen atoms belonging to three water molecules, two bidentate acetate groups and two bridging acetate groups. The coordination geometry at Pb is a monocapped square antiprism.[viii] [nine] The trihydrate thermally decomposes to a hemihydrate, Lead(OAc)2·½H2O, and to basic acetates such as PbfourO(OAc)6 and PbtwoO(OAc)2.[7]
| Degree of hydration | Pb coordination | Strongly bonded assemblage | Weakly bonded aggregation |
|---|---|---|---|
| Anhydrous[7] Atomic number 82(OAc)ii |  |  2D canvas | 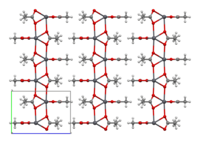 sheets stacked with hydrophobic surfaces in contact |
| Trihydrate[8] [nine] Pb(OAc)two·3HtwoO |  |  1D concatenation | 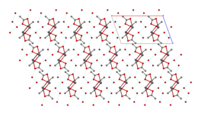 chains linked past hydrogen bonds |
Uses [edit]
Sweetener [edit]
Similar other lead(2) salts, atomic number 82(II) acetate has a sweet sense of taste, which led to its historical use as a saccharide substitute in both wines and foods.[ten] The ancient Romans, who had few sweeteners also honey, would boil must (grape juice) in lead pots to produce a reduced sugar syrup chosen defrutum, concentrated again into sapa. This syrup was used to sweeten wine and to sweeten and preserve fruit. It is possible that atomic number 82(Two) acetate or other lead compounds leaching into the syrup might have acquired pb poisoning in those who consumed it.[xi] Lead acetate is no longer used in the production of sweeteners because of its recognized toxicity. Mod chemical science tin can easily detect it, which has almost completely stopped the illegal apply that connected decades after its apply as a sweetener was banned.[12]
Historical incidents [edit]
The primeval confirmed poisoning by lead acetate was that of Pope Clement Ii who died in Oct 1047. A toxicological examination of his remains conducted in the mid-20th century confirmed centuries-onetime rumors that he had been poisoned with lead sugar.[13] It is not clear whether he was assassinated.
In 1787 painter and biographer Albert Christoph Dies swallowed, past accident, approximately 3 / iv oz (20 g) of lead acetate. His recovery from this poison was wearisome and incomplete. He lived with illnesses until his death in 1822.[14] [fifteen]
Although the apply of lead(2) acetate as a sweetener was already illegal at that time, composer Ludwig van Beethoven may accept died of lead poisoning acquired past wines adulterated with pb acetate (see likewise Beethoven's liver).[xvi] [17]
In the 1850s, Mary Seacole applied pb(II) acetate, amid other remedies, confronting an epidemic of cholera in Panama.[18] [nineteen]
In 1887, 38 hunting horses belonging to Captain William Hollwey Steeds were poisoned in their stables at Clonsilla House, Dublin, Ireland. At least x of the hunters died. Captain Steeds, an 'extensive committee amanuensis', had previously supplied the horses for the Bray and Greystones Jitney. It transpired they had been fed a bran brew that had been sweetened with a toxic lead acetate.[20]
Cosmetics [edit]
Lead(II) acetate, besides every bit white atomic number 82, has been used in cosmetics throughout history.[21]
Until recently,[ when? ] it was still used in the USA in men's hair colouring products[22] similar Grecian Formula. It was not until just a few years ago[ when? ] when the manufacturer removed lead acetate from the hair coloring product, and equally of July 2018 the ingredients in Grecian Formula are h2o, isopropyl booze, triethanolamine, bismuth citrate, sodium thiosulfate, fragrance, and panthenol. Lead acetate has been replaced by bismuth citrate as the progressive colorant. Its employ in cosmetics has been banned in Canada by Health Canada in 2005 (effective at the finish of 2006) based on tests showing possible carcinogenicity and reproductive toxicity,[23] and information technology is also banned in the European Wedlock[23] and has been on the California Proposition 65 warning list as a carcinogen since 1988.[24]
Medical uses [edit]
Pb(II) acetate solution was a commonly used folk remedy for sore nipples.[25] In modernistic medicine, for a time, it was used as an astringent, in the course of Goulard's Excerpt, and it has too been used to treat poison ivy.[26]
Industrial uses [edit]
Lead(Two) acetate paper is used to detect the poisonous gas hydrogen sulfide. The gas reacts with lead(2) acetate on the moistened test paper to course a grayness precipitate of lead(2) sulfide.
An aqueous solution of pb(Ii) acetate is the byproduct of a 1:ane ratio of hydrogen peroxide and white vinegar (acetic acid) used in the cleaning and maintenance of stainless steel firearm suppressors (silencers) and compensators. The solution is agitated by the bubbles action of the hydrogen peroxide, and the principal reaction is the dissolution of pb deposits within the suppressor by the acetic acid, which forms lead acetate. Because of its high toxicity, this chemical solution must exist appropriately disposed by a chemical processing facility or hazardous materials middle. Alternatively, the solution may be reacted with sulfuric acid to precipitate nearly insoluble lead(II) sulfate. The solid may then exist removed by mechanical filtration and is safer to dispose of than aqueous atomic number 82 acetate.
It was also used in making of slow matches during the Middle Ages. It was made past mixing natural form of lead(II) oxide called litharge and vinegar.
Carbohydrate of lead was a recommended agent added to linseed oil during heating to produce "boiled" linseed oil, the pb and heat acting to crusade the oil to cure faster than raw linseed oil.[27]
See also [edit]
- Saturn'due south Tree
References [edit]
- ^ a b c d e Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. ISBN0-07-049439-8.
- ^ a b c d e "Lead(2) acetate".
- ^ a b c "Lead(2) acetate trihydrate".
- ^ a b c d Sigma-Aldrich Co., Lead(II) acetate trihydrate. Retrieved on 2014-06-08.
- ^ "Lead compounds (as Pb)". Immediately Unsafe to Life or Health Concentrations (IDLH). National Found for Occupational Safety and Wellness (NIOSH).
- ^ "Pb Acetate in 'Progressive' Hair Dye Products". fda.gov. 7 October 2021.
- ^ a b c Martínez-Casado, Francisco J.; Ramos-Riesco, Miguel; Rodríguez-Cheda, José A.; Cucinotta, Fabio; Matesanz, Emilio; Miletto, Ivana; Gianotti, Enrica; Marchese, Leonardo; Matěj, Zdeněk (2016). "Unraveling the Decomposition Process of Lead(II) Acetate: Anhydrous Polymorphs, Hydrates, and Byproducts and Room Temperature Phosphorescence". Inorg. Chem. 55 (17): 8576–8585. doi:10.1021/acs.inorgchem.6b01116. PMID 27548299.
- ^ a b Rajaram, R. K.; Mohana Rao, J. Grand. (1982). "Crystal structure of pb acetate trihydrate". Z. Kristallogr. 160 (1–iv): 225–233. doi:10.1524/zkri.1982.160.14.225. S2CID 201671682.
- ^ a b Bryant, Robert M.; Chacko, V. P.; Etter, Margaret C. (1984). "Carbon-xiii CP/MAS NMR and crystallographic investigations of the structure and solid-state transformations of atomic number 82(II) acetate trihydrate". Inorg. Chem. 23 (22): 3580–3584. doi:ten.1021/ic00190a029.
- ^ "The Disturbingly Long History of Lead Toxicity in Winemaking." Anna Archibald, thirty July 2020. Retrieved: 22 December, 2020.
- ^ Lead Poisoning and Rome
- ^ Stoeppler, M. (1992), Hazardous Metals in the Environment, Techniques and Instrumentation in Analytical Chemistry, vol. 12, Elsevier, p. 60, ISBN9780080875606,
From the results achieved so far it is obvious that the purity law for pb in wines in the last 2 centuries was frequently ignored.
- ^ Specht W and Fischer Yard (1959). Vergiftungsnachweis an den Resten einer 900 Jahre alten Leiche. Arch. Kriminol., 124: 61-84. [Translation:Intoxication bear witness in the remains of a 900-yr-old corpse]
- ^ I or more of the preceding sentences incorporates text from a publication now in the public domain:Chisholm, Hugh, ed. (1911). "Dies, Christoph Albert". Encyclopædia Britannica. Vol. eight (11th ed.). Cambridge Academy Press. p. 211.
- ^ Dies, Albert Christoph (1810). Biographische Nachrichten von Joseph Haydn nach mündlichen Erzählungen desselben entworfen und herausgegeben [Biographical Accounts of Joseph Haydn, written and edited from his own spoken narratives]. Vienna: Camesinaische Buchhandlung. English translation in: Dies, Albert Christoph (1963). "Biographical Accounts of Joseph Haydn". In Gotwals, Vernon (ed.). Haydn: Two Contemporary Portraits. (translation past Vernon Gotwals). Milwaukee: Univ. of Wisconsin Press. ISBN0-299-02791-0.
- ^ "Beethoven und Blei: Tödliches Zusammenspiel". Archived from the original on 2009-02-21. Retrieved 2009-09-12 .
- ^ "Beethoven litt unter Bleivergiftung". Archived from the original on 2009-02-21. Retrieved 2020-02-24 .
- ^ Mary Seacole: Wonderful Adventures of Mrs. Seacole in Many Lands, Affiliate Iv, (1990 Oxford University Press reprint) ISBN 0-19-506672-3; (2005 Penguin 20th Century Classics reprint, ed. Sarah Salih) ISBN 0-fourteen-043902-ane
- ^ Jane Robinson: Mary Seacole: The Charismatic Blackness Nurse who became a heroine of the Crimea, p.53. Constable 2004 (p/b. ISBN 1-84119-677-0)
- ^ Weekly Irish Times, Saturday 15 October 1887; Enniskillen Chronicle and Erne Packet, 24 Oct 1887, p. iii
- ^ Gunn, Fenja. (1973). The Artificial Face: A History of Cosmetics. — as cited in Leisure Activities of an 18th Century Lady
- ^ Pb Based Hair Products: Too Chancy for Household Employ - Results, Howard W. Mielke, PhD, Myiesha D. Taylor, Chris R. Gonzales, One thousand. Kelley Smith, Pamela V. Daniels, and Ayanna V.Buckner. Periodical of American Pharmaceutical Association (NS37, Jan/Feb 1997:85-89).
- ^ a b "Can West News Service: Grecian Formula in a grey zone subsequently ban". Archived from the original on 2007-08-08.
- ^ "The Proffer 65 Listing". Archived (PDF) from the original on 2014-ten-31. Retrieved 2014-eleven-01 .
- ^ The American Frugal Housewife, past Lydia M. Child
- ^ Laboratory transmission in biological science. Sharpe. 1911, American Book Company. p. 351
- ^ Andés, Louis Edgar, and Arthur Morris. Oil colours and printers' inks a practical handbook treating of linseed oil, boiled oil, paints, artists' colours, lampblack and printers' inks, blackness and coloured. London: Scott, Greenwood. 1903. 41. Print.
External links [edit]
- Case Studies in Environmental Medicine - Lead Toxicity
- Essay on "Lead Poisoning and Rome"
- HowStuffWorks "What Kind of Pilus Color Do Men Use?" give-and-take of progressive dyes containing lead acetate
- National Pollutant Inventory - Atomic number 82 and Lead Compounds Fact sail (Does Not Bring Up Lead)
- ToxFAQs: Lead
- Usa Food and Drug Administration (FDA) fact canvass "Lead Acetate in Pilus Dye Products"
- U.s. Food and Drug Administration (FDA)21CFR73.2396 "PART 73 -- LISTING OF COLOR ADDITIVES EXEMPT FROM CERTIFICATION, Subpart C--Cosmetics, Sec. 73.2396 Atomic number 82 acetate"
Aqueous Lead Ii Nitrate Formula,
Source: https://en.wikipedia.org/wiki/Lead(II)_acetate
Posted by: alanishispout80.blogspot.com



0 Response to "Aqueous Lead Ii Nitrate Formula"
Post a Comment